Minor Pests of Honey Bees in Mississippi
For the purpose of this publication, honey bee pests are classified as major or minor pests based on whether they cause chronic damage to bees, whether they vector disease pathogens, and, of course, how prevalent they are. In certain cases, the distinction is somewhat vague; for example, many Mississippi beekeepers consider small hive beetles to be a major economic nuisance, but from a biological standpoint, they are considered a minor pest because they are not known to vector disease or cause chronic damage to colonies (bees will abscond if beetle pressure becomes too great). Varroa mites and tracheal mites are currently the only major honey bee pests in North America. However, all honey bee pests are noteworthy because any added stress to bee colonies can cause bees to abscond, slow brood production, or even disrupt the social hierarchy of the hive.

Wax Moths, Greater and Lesser
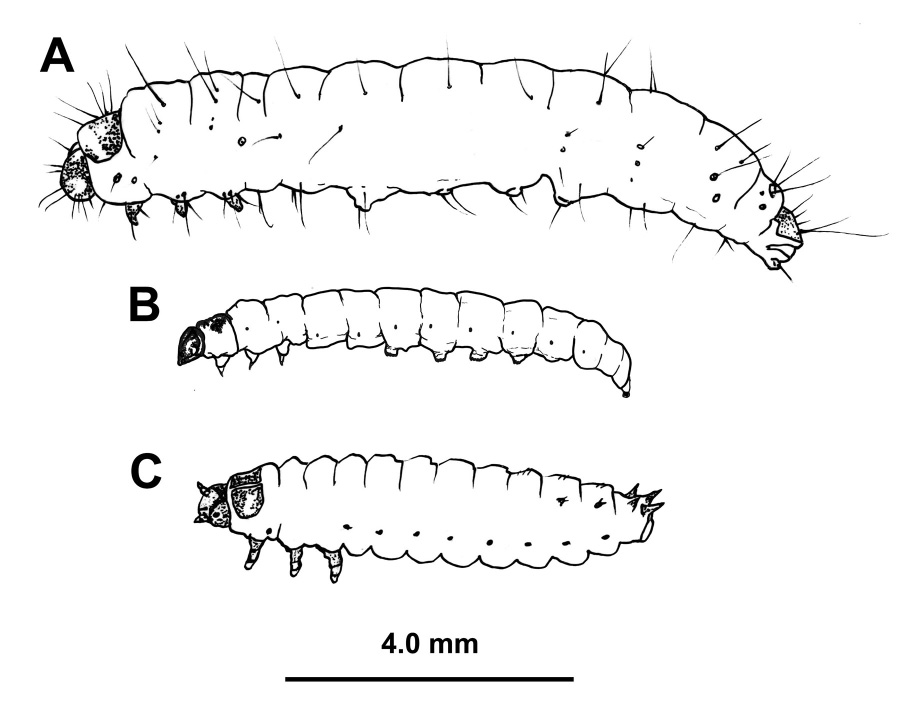
These ubiquitous insects belong to a large family of moths commonly known as “snout moths.” The larvae of wax moths—called waxworms—are produced in the commercial pet industry as feeder insects for reptiles; they are also commonly used as fishing bait. In some Asian countries, such as Thailand, waxworms constitute an important part of the human diet as a source of high-quality protein. There are two species of wax moth that are associated with honey bee colonies: the greater wax moth (Galleria melonella) and the lesser wax moth (Achroia grisella) (Figure 1). Both occur in Mississippi, but the larvae of the greater wax moth are responsible for most of the damage done to stored combs and woodenware.
Damage
The larva stage is the only destructive life stage of the wax moth; the adults do not feed. Eggs are laid in clumps in the cracks and crevices of both the exterior and interior of the hive. Female moths fly at dusk and will enter an inactive hive to oviposit. Within 5 to 8 days, the newly hatched larvae will seek out comb and begin feeding. They are particularly attracted to brood comb, which contains nutritious pollen and bee bread, but they do not feed on live honey bee pupae.
Young wax moth larvae will often escape a beekeeper’s notice, due to their habit of tunneling underneath the cappings of brood comb; this is more common with lesser wax moths. They usually travel in a straight line across the comb, leaving a trail of silk webbing and frass (bug feces) in their wake. Honey bees will systematically uncap bee pupae whose cells have been invaded by wax moth larvae until they find the perpetrator and destroy it or carry it out of the hive. The uncapped pupae are referred to as “bald brood,” and the sight can be alarming to a new beekeeper. This is no cause for worry, however. Unless the pupae have been damaged, they will be recapped.
Treatment
Combs from dead or absconded colonies should be immediately picked up to prevent subsequent wax moth outbreaks in the apiary. The combs can be stored in a freezer (0°F) for 48 hours to kill any unseen larvae or eggs, and then placed back in their boxes and stacked on a solid floor. Wax moths are excellent infiltrators of stored equipment, so it is strongly recommended that combs be treated prophylactically with an insecticide after freezing to prevent subsequent outbreaks during storage. There are two recommended options for treating stored comb: one is a chemical fumigant, and the other is a biological treatment.
Chemical fumigation is the most cost-effective and least labor-intensive, but you will need to have access to some sort of enclosed space that will not allow fumes to leak into the environment. It is important to make your stack as airtight as possible for fumigation—about five boxes high and all the joints sealed with tape. Wax moth crystals, which contain the active ingredient paradichlorobenzene (PDB), produce a fumigant that is heavier than air, so they should be applied to the frame bars in the top box and covered with a lid. Be sure to follow the recommended insecticide rate for the number of boxes you are treating to get the most effective control. Do not use “moth balls” containing the active ingredient naphthalene; this chemical readily leaches into honey and renders it unsuitable for human consumption.
The biological insecticide B401 contains a Bacillus thuringiensis that specifically targets moth larvae. It can be applied to comb after the honey harvest and will remain active for months. Larvae must ingest B401 for it to be fatal, so, while it is not a feeding deterrent, it will prevent new generations of wax moths in stored equipment. This product is nontoxic to bees and humans and is also registered for use with certified organic products.
Hives that contain wax moth pupae must be cleaned by manually removing the cocoons and webbing. These will be found nestled into the interior wooden parts of the hive.
Young wax moth larvae are often confused with the larvae of the small hive beetle (Figure 2). Both are dirty white with brown head capsules. Co-infestations can and do occur, usually in a very weak colony or following an absconding event, but wax moths are far more common in dead colonies. There is a simple rule of thumb to differentiate between wax moth and hive beetle larvae that does not require a closer look at the specimen: if it squishes easily when rolled between thumb and forefinger, it’s a wax moth.
Small Hive Beetles
The small hive beetle (Aethina tumida Murray) is the newest pest of honey bees in North America. Although many southern beekeepers would argue that this is an active predator of honey bee colonies, it is actually more of an opportunistic pest that takes advantage of colonies that are already weak. Adult beetles are attracted to hive odors, particularly to the honey bee alarm pheromone, isopentyl acetate (Figure 3). This chemical is responsible for the “banana smell” that you sometimes encounter when opening a hive. The beetle is also attracted to the odor of a hopelessly queenless hive, most likely because queenlessness causes an increase in alarm pheromone.
Figure 3. Adult small hive beetle. Drawing by Joe A. MacGown.
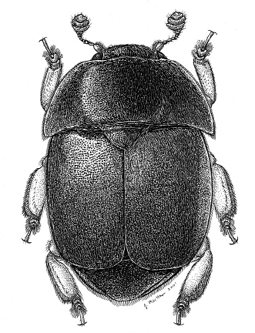
The presence of adult beetles in a colony is not a reliable indicator of colony failure. Hundreds of adults can inhabit a strong, healthy hive without undergoing significant reproduction. In this case, adult beetles tend to congregate in honey supers and do little more than aggravate worker bees as they move about the hive. There is often low-level beetle reproduction in both strong and weak hives, but bees with hygienic tendencies will eliminate eggs and larvae before they become too numerous.
Damage
Small hive beetle adults feed on pollen and honey but not to a degree that would affect the bee colony. The larva stage is the damaging life stage. When larvae are present in high numbers in a weak honey bee colony, the honey bees usually abscond, leaving the beetle larvae free to devour honey, bee bread, and brood.
The feeding and defecation from beetle larvae cause the honey to ferment; this is because the larvae carry a yeast in and on their bodies. The fermentation odors subsequently attract more hive beetle adults to the colony, and within a week, the combs will be a slimy mess. A colony that has been “slimed out” by small hive beetle larvae usually has an odor of rotten oranges that can be detected several yards away. Hives that have a solid bottom board may have fermented honey running out the front entrance.
When small hive beetle larvae have matured (approximately 7 days post-hatch), they will exit the hive and burrow in the ground surrounding the colony. This usually occurs at dusk, so if you discover a hive that has been slimed, it is safe to assume some larvae have infested your apiary soil. It is wise to either treat the soil surrounding the hives with insecticide or relocate your hives a few miles away to prevent a reinfestation from small hive beetles.
Once a slime-out has occurred in an apiary and larvae have successfully entered the soil, that apiary has become a reservoir for small hive beetles. It takes about 3 years for the population of hive beetles to build up to critical levels if no control measures are taken; at this point, hives must be relocated.
Treatment
There is only one chemical insecticide that is registered in Mississippi for in-hive small hive beetle control: the coumaphos-based insecticide CheckMite Plus. Coumaphos has been shown to have deleterious effects on honey bee queens, so this treatment is not highly recommended; however, if you do use it, it is imperative that you follow treatment protocol for small hive beetles to minimize any harmful effects it may have on your bees.
Many mechanical control measures are very low risk to honey bees but quite effective at trapping or killing hive beetle adults. These include in-hive reservoir traps, bottom board traps, entrance traps, refuge traps containing diatomaceous earth or boric acid (again, care must be taken to prevent bees from coming into contact with these substances), and entanglement traps. Do not use roach motels containing the active ingredient fipronil. This insecticide is highly toxic to honey bees and may be spread throughout the hive when small hive beetles come into contact with it.
A ground drench application of permethrin is the safest chemical treatment for hive beetle larvae that have burrowed and pupated. See MSU Extension Publication 2825 Small Hive Beetle (http://extension.msstate.edu/publications/small-hive-beetle) for more information about chemical treatments and traps.
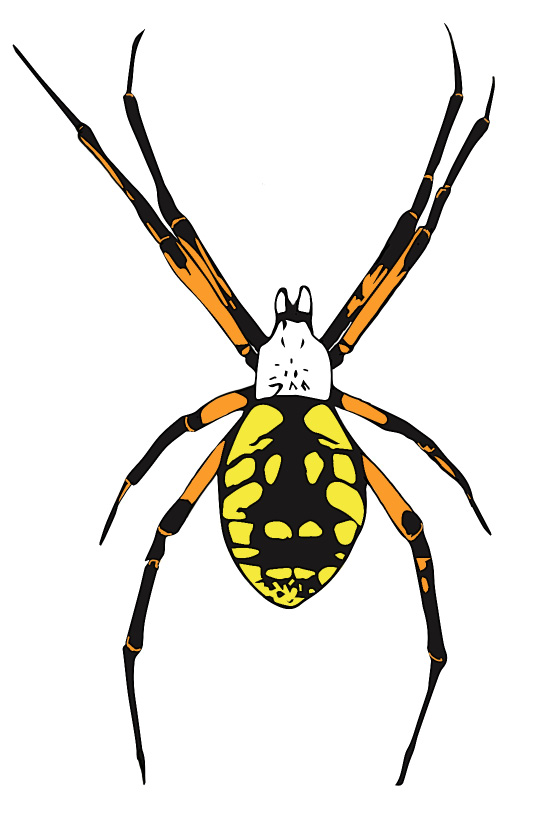
Yellow Garden Spiders
The yellow garden spider (Argiope aurantia) is also called the “writing spider” because of the zig-zag stabilimentum it builds into the center of its web. Females of this species are often seen on or between beehives on sturdy webs (Figure 4). This spider is considered a garden beneficial, but in the apiary, it is a nuisance that ensnares and devours honey bees. Webs are often built between adjacent hives or between hives and nearby vegetation. The venom of this spider is not harmful to humans, nor are these spiders likely to bite if disturbed. However, the large size (up to 11/8 inch, not including leg span) attained by females can be quite intimidating to beekeepers. The spiders spin new webs every day, and not always in the same place, but they tend to stay put in an apiary once they’ve established, so pulling down a web does not encourage them to relocate. Relatively few bees are consumed by individual yellow garden spiders, and the webs seldom obstruct major flyways.
Argentine Ants
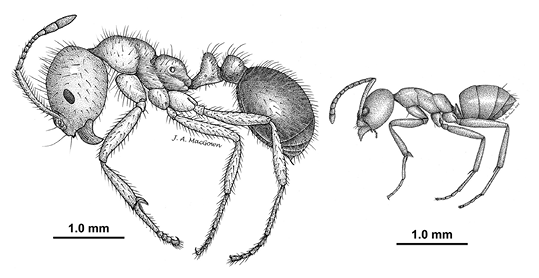
Mississippi beekeepers will likely encounter Argentine ants (Linepithema humile) in their apiaries at some point because these ants have a preference for honey bees. Worker ants feed on brood, dead bees, and honey stores, but they do not typically establish in the hive. In large beekeeping operations where individual colonies may not receive much attention, ant populations can build up quickly. Heavy infestations can cause honey bees to abscond.
These ants are commonly mistaken for the equally ubiquitous red imported fire ant (Solenopsis invicta); however, Argentine ants do not have stingers (Figure 5). They have been found to carry a strain of deformed wing virus, but it is not known whether this strain affects honey bees. Argentine ants are not a threat to strong colonies, and they do an excellent job of cleaning out deadout colonies. But, to prevent ants from taking down a small or weak colony, you can create a physical barrier with a sticky substance such as petroleum jelly or Tangle-Trap, or place the feet of your hive stand in shallow containers of oil.
Vertebrates
Skunks, mice, and bears are the most frequently encountered vertebrate pests of North American apiaries, though bears are seldom troublesome in Mississippi. However, in regions where bears do occur, their presence is made known immediately to beekeepers. Bears are “colony raiders,” knocking over boxes and breaking equipment to get to the brood comb within, and they can cause significant economic damage to a beekeeping business. Electric fences are usually used around apiaries in bear territory to prevent this devastating damage.
Unlike bears, skunks do not feast on bee brood, but on the adult foragers and guard bees. Being nocturnal hunters, they are seldom caught in the act, though their handiwork is unmistakable: scratch marks on the front of the hive or landing board and piles of chewed-up bee carcasses, called “cuds,” on the ground. Skunks tend to be repeat offenders, and, although the damage they cause to beekeeping equipment is minimal, their frequent nightly attacks can put colonies on edge. It is believed that they target the most defensive colonies in the apiary, rather than systematically raiding all the hives, because the most defensive bees will recruit the most guards to the hive entrance. Over time, skunk attacks can weaken a strong colony, so it is best to address the problem as soon as it becomes evident.
The best control measure for nuisance skunks is prevention. Raising your colonies off the ground more than 18 inches will discourage skunks from harassing your bees. If you have a nuisance skunk already visiting your apiary, you can contact the United States Department of Agriculture Wildlife Services and request that the skunk be trapped and relocated. Licensed hunters in Mississippi may legally remove skunks from their property at any time, in accordance with the Mississippi Department of Wildlife, Fisheries, and Parks Rule 7.1.
Mice are most commonly encountered nesting in the bottoms of hives, especially during the cool seasons. While they do not feed on bees, they do chew woodenware and comb wax, and they make a general mess of the hive interior. Installing a mouse guard (a panel of 6-mesh galvanized hardware cloth stapled over the hive entrance) after you have winterized your hives is a cheap and easy way to prevent costly mouse damage.
The information given here is for educational purposes only. References to commercial products, trade names, or suppliers are made with the understanding that no endorsement is implied and that no discrimination against other products or suppliers is intended.
Publication 3195 (POD-04-24)
By Audrey B. Sheridan, Extension/Research Associate III, Biochemistry, Molecular Biology, Entomology, and Plant Pathology.
The Mississippi State University Extension Service is working to ensure all web content is accessible to all users. If you need assistance accessing any of our content, please email the webteam or call 662-325-2262.


